
With a bit of practice, you will then be able to say whether 2 different representations of a chemical correspond to the same compound, enantiomers or something else.
NEWMAN PROJECTION CHEMDOODLE HOW TO
That is why it is important to be able to represent molecules in 3D using different representations, but also to understand how to draw one representation of a molecule starting from another.
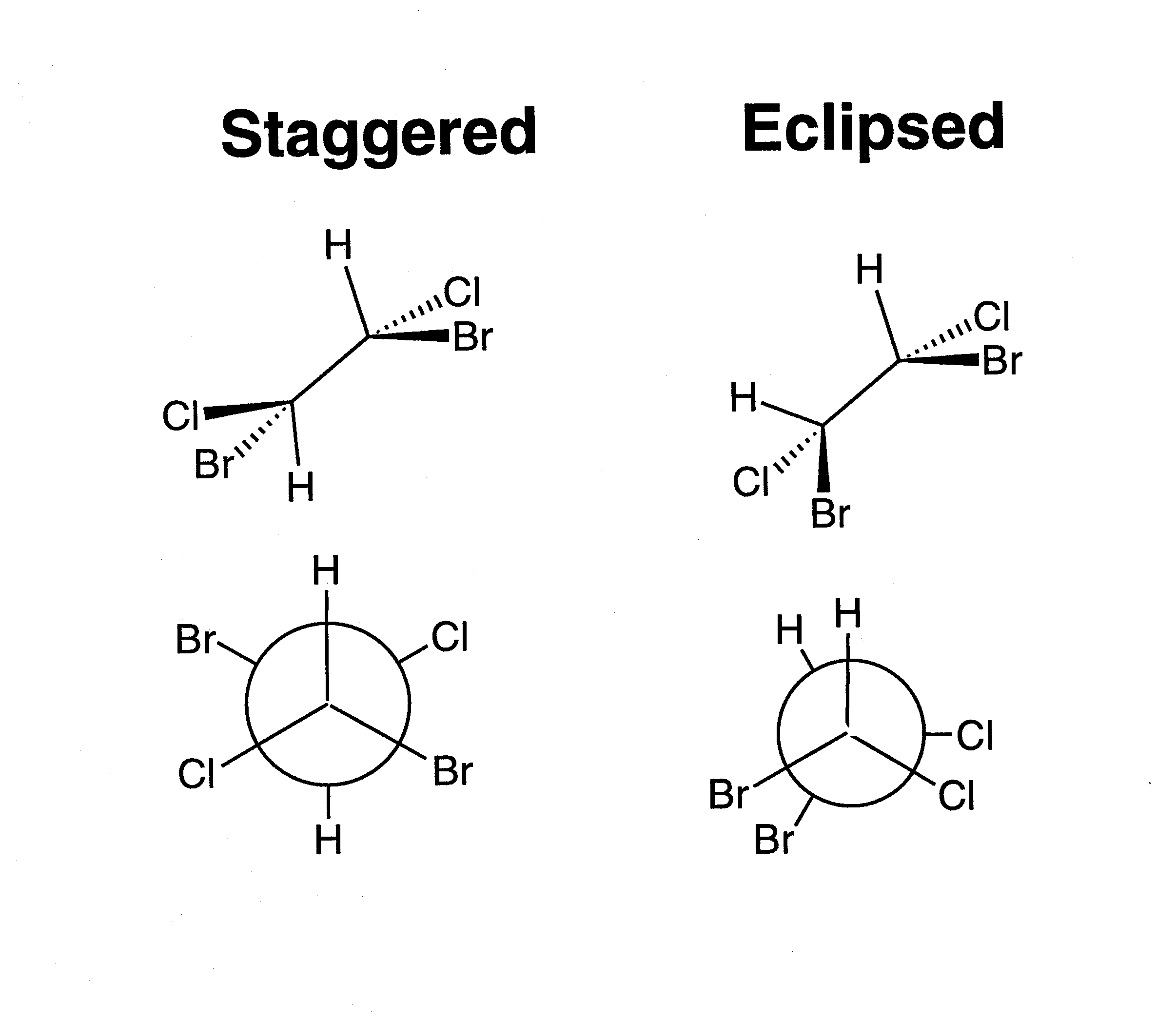
As example, see below how 2 enantiomers of 2 - Butanol are represented using Sawhorse and Newman projections: at university, you will learn that compounds can exist in different conformations and that chiral carbons can have 2 different configurations that you will learn to assign using 3D representations. If you look at the Newman projection, you can see that it is a compact version of the Sawhorse projection. Sustituents attached to front carbon all have bonds starting from a dot while substituents attached to back carbon have bonds starting from a circle. Front carbon is represented as dot and back carbon as circle. In Newman projection, we look at carbon - carbon bond. * Please keep in mind that all text is machine-generated, we do not bear any responsibility, and you should always get advice from professionals before taking any actions * Please keep in mind that all text is machine-generated, we do not bear any responsibility, and you should always get advice from professionals before taking any actions. So we have a 5 - carbon chain with methyl group at c - 3. C - 3 has methyl group attach, while c - 4 has only h atoms. Then the front carbon in the circle is c - 3 and the hidden carbon is c - 4. Let's call terminal c of ethyl group c - 1. In front of carbon, ethyl group has the longest chain, with another two c atoms. There is one c atom at the centre of the circle and a second one hidden at back of the circle. Find the longest continuous chain of carbon atoms. What is its bond - line notation? Step 1. Here is Newman projection of hydrocarbon. Since bond line notations don't show hydrogen atoms attached to carbon atoms, bond line notation for ethane will just be straight line. Here's how two attached propellers would look. If you draw two carbon atoms as you d do for bond line notation, you d get a line - this line represents your barrel. Back carbon will also have three h atoms attach: one to the left, one to the right, and one pointing straight down. It has three h atoms attach: one to the left, one to the right, and one straight up. Atoms that point straight down will be coming towards your point of view, while atoms that point straight up will be further away from your point of view. Atoms that you see coming out to the right of the circle will be coming out of the plane of the page. Atoms you can see coming out of the circle to the left will go into the plane of the page. One closer will represent front carbon, while one further away towards the tip of the barrel will be back carbon.
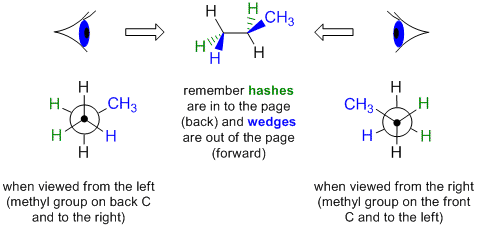
For some reason, you 've got two propellers attached to the barrel - one closer to you, one further away.

This will represent the plane of the page. Now, imagine you re looking down barrel of gun. As you can see, only hydrogen atoms are attached to these two carbon atoms, so molecule must obviously have 2 carbon atoms. These two carbon atoms that form bond are drawn as an intersection of three black lines - this is the front carbon atom - and a large black circle - this is back carbon. So, start with the staggered Newman projection for ethane, or c_2 h_6, which looks like this molecule can have no less than 2 carbon atoms, SInce Newman projections are made by looking down bond of carbon atoms.


 0 kommentar(er)
0 kommentar(er)
